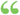 Oj +± 2H2O + 2C12 + 28,000 calories. and in this action raising the temperature drives the equilibrium backwards, and a lowering in the temperature is required to increase the yield of chlorine. The rule is obvious, and applies to all reversible reactions... Oj +± 2H2O + 2C12 + 28,000 calories. and in this action raising the temperature drives the equilibrium backwards, and a lowering in the temperature is required to increase the yield of chlorine. The rule is obvious, and applies to all reversible reactions...  General Chemistry for Colleges - Page 183by Alexander Smith - 1916 - 662 pagesFull view General Chemistry for Colleges - Page 183by Alexander Smith - 1916 - 662 pagesFull view - About this book
 | Alexander Smith - 1917 - 954 pages
...2H2O + 2C12 + 28,000 cal., and in this action raising the temperature drives the equilibrium backward, and a lowering in the temperature is required to increase...be written with a negative heat of reaction (eg, p. 100), the heat can, of course, be transferred to the other side with its sign changed. This law is... | |
 | Alexander Smith - 1917 - 958 pages
...equilibrium twickward, and a lowering in the temperature is required to increase the yield of chlorine. the temperature of a system in equilibrium is raised,...sign) in the equation. If the equation happens to l)e written with a negative heat of reaction (eg, p. 100), the heat can, of course, be transferred... | |
 | Alexander Smith - 1922 - 608 pages
...interaction of hydrogen chloride and oxygen liberates heat. 4HC1 + O, <=± 2H2O + 2C1, + 28,000 cal. aiid in this action raising the temperature drives the...written with a' negative heat of reaction (eg, p. 162), the heat can, of course, be transferred to the other side with its sign changed. This law is... | |
 | Edward Everett Bugbee - 1922 - 276 pages
...Pb = 3FeO + PbO - 22,900 cal. According to van't Hoff's law, when the temperature of such a system is raised, the equilibrium point is displaced in the direction which absorbs heat, that is to say, the above reactions will proceed in a right-handed direction. Ferric oxide is 'soluble... | |
 | Edward Everett Bugbee - 1922 - 274 pages
...Pb = 3FeO + PbO - 22,900 cal. According to van't Hoff's law, when the temperature of such a system is raised, the equilibrium point is displaced in the direction which absorbs heat, that is to say, the above reactions will proceed in a right-handed direction. Ferric oxide is soluble... | |
 | Alexander Smith, James Kendall - 1926 - 1088 pages
...interaction of hydrogen chloride and oxygen liberates heat, 4HC1 + Oj +± 2H2O + 2C12 + 28,000 calories. and in this action raising the temperature drives...be written with a negative heat of reaction (eg, p. 297), the heat can, of course, be transferred to the other side with its sign changed. This law is... | |
 | United States. Army. Chemical Corps - 1940 - 168 pages
...2C12 + 28,400 c alories and in this action raising the temperature drives the equilibrium backward, and a lowering in the temperature is required to increase...is displaced in the direction which absorbs heat. This is known as " Van't Hoff's law of mobile equilibrium" and it will be seen that it is only a specific... | |
 | United States. Army. Chemical Corps - 1940 - 180 pages
...4HCl+O2;=±2H2O+2Cl2+28,400 calories and in this action raising the temperature drives the equilibrium backward, and a lowering in the temperature is required to increase...is displaced in the direction which absorbs heat. This is known as " Van't Hoff's law of mobile equilibrium" and it will be seen that it is only a specific... | |
 | United States. Army. Chemical Corps - 1940 - 176 pages
...equilibrium backward, and a lowering in the temperature is required to increase the yield of cldorine. The rule is obvious and applies to all reversible...is displaced in the direction which absorbs heat. This is known as " Van't Hoff's law of mobile equilibrium" and it will be seen that it is only a specific... | |
 | United States. War Department - 202 pages
...2C12 + 28,400 calories and in this action raising the temperature drives the equilibrium backward, and a lowering in the temperature is required to increase...is displaced in the direction which absorbs heat. This is known as " Van't Hoff's law of mobile equilibrium" and it will be seen that it is only a specific... | |
| |