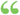 For compressible flow this becomes: where y is the ratio of the specific heat at constant pressure to that at constant volume... For compressible flow this becomes: where y is the ratio of the specific heat at constant pressure to that at constant volume...  Science - Page 66edited by - 1883Full view Science - Page 66edited by - 1883Full view - About this book
 | Alexander Graham Clark - 1911 - 472 pages
...heat of a gas ? Show that the value of n in an equation of the curve for an adiabatic expansion is the ratio of the specific heat at constant pressure to that at constant volume. (7) Combine Boyle's and Charles's laws. CHAPTER VIII HORSE-POWEK 82. What is Horse-power ? — -Considerable... | |
 | 1912 - 612 pages
...to be vaporized. For potassium vapor having a temperature between 950° C. and 1000° U, the ratio y of the specific heat at constant pressure to that at constant volume was found experimentally to be T64. From 880°-920°, y = 1'69 ; from 700°-730°, y = 1'63 ; and from... | |
 | Henry Enfield Roscoe, Carl Schorlemmer - 1913 - 1496 pages
...identity of atom and molecule has been confirmed in the case of mercury vapour by the determination of the ratio of the specific heat at constant pressure to that at constant volume (see Vol. I., p. 135). In the same way it has been shown that the molecules of sodium and potassium... | |
 | 1914 - 458 pages
...-points were calculated from the formula Tl = ( JXi )r ' , Vj, V2, and Tt being determined exTI VV / of the specific heat at constant pressure to that at constant volume, was taken as i • |, all the gases used being diatomic. The main sources of error are discussed, and... | |
 | George Downing Liveing, Sir James Dewar - 1915 - 646 pages
...of compression to a like amount will increase the temperature by nearly two-thirds, in consequence of the ratio of the specific heat at constant pressure to that at constant volume being now 1'66. It is not so easy to explain why the bright band should appear while the sodium is... | |
 | Harold Albert Wilson - 1915 - 428 pages
...finding X and X' the velocity of sound in the gas can be determined. The equation v = */ — enables the ratio of the specific heat at constant pressure to that at constant volume for any gas to be calculated when v is known. Kundt's apparatus is often used to find the value of... | |
 | United States. National Advisory Committee for Aeronautics - 12 pages
...p/p0=(p/Po)'r comes to rest adiabatically the stop pressure is as shown in hydrodynamics, y=Cp/Cv being the ratio of the specific heat at constant pressure to that at constant volume. This formula is valid for engineering speeds below that of sound in the fluid; for higher and for extremely... | |
 | Samuel Sheldon, Erich Hausmann - 1917 - 156 pages
...values are substituted in equation (4;, which can be rewritten in the form in order to find the ratio 7 of the specific heat at constant pressure to that at constant volume. APPARATUS. — Kundt's tube with metal rod, toothed wheel driven by a motor rotator, motor rheostat,... | |
 | Friedrich Rudolf Schenck - 1919 - 266 pages
...1100° 1300° 1500° Vapor Pressure of the Metals Hg , Cd , Zn and B i FIG. 1. that for a monatomic gas the ratio of the specific heat at constant pressure to that at constant volume must have a definite value, namely 1.667. Kundt and Warburg f found the value 1.666 for mercury vapor.... | |
 | William Watson - 1920 - 590 pages
...Now in § 80 (equation 80) we saw that under adiabatic conditions the elasticity was yP, where y is the ratio of the specific heat at constant pressure to that at constant volume. The question therefore arises whether in the case of soundwaves in a gas the changes of pressure are... | |
| |