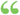 For compressible flow this becomes: where y is the ratio of the specific heat at constant pressure to that at constant volume... For compressible flow this becomes: where y is the ratio of the specific heat at constant pressure to that at constant volume...  Science - Page 66edited by - 1883Full view Science - Page 66edited by - 1883Full view - About this book
 | International Correspondence Schools - 1907 - 456 pages
...That is, the product of the pres- A sure and the £th power of the volume is constant, in which k is the ratio of the specific heat at constant pressure to that at constant volume. As stated in Art. 62, k = 1.405 for dry air. 99 SS SO 79 70 90 '66 40 ao 78. Suppose that 10 cubic... | |
 | Sydney Young - 1908 - 460 pages
...atomic weight is 200. The velocity of sound in mercury vapour was determined by Kundt and Warburg, and the ratio of the specific heat at constant pressure to that at constant volume was found to be 1-66. From this it follows (p. 188) that there is no increase of internal energy of... | |
 | Alfred James Herrick, Frederic Anthony Buechel - 1909 - 64 pages
...expansion for in such an expansion pv is a constant where p is the pressure, v the volume of the gas and y the ratio of the specific heat at constant pressure to that at constant volume, Hence, pv = k ( a constant ) We know that, pv-R6. where 6 is the absolute temperature and R a constant.... | |
 | 1910 - 1002 pages
...the isothermal elasticity, required for calculating the velocity of sound, is therefore the same as the ratio of the specific heat at constant pressure to that at constant volume. 12. Expérimentai Verification of ike Ratio of Specific Heats. — This was a most interesting and... | |
 | Hugh Chisholm - 1910 - 1006 pages
...the isothermal elasticity, required for calculating the velocity of sound, is therefore the same as the ratio of the specific heat at constant pressure to that at constant volume. 12. Experimental Verification of the Ratio of Specific Heats. — This was a most interesting and important... | |
 | William Kent - 1910 - 1620 pages
...— pressure outside orifice. i — specific volume of gas inside orifice in cu. ft. per ib. y — ratio of the specific heat at constant pressure to that at constant volume. lr. where -r -- 1.404, we have for a circular orifice of diameter d . the initial temperature of the... | |
 | Frank Henley Leeds, William John Atkinson Butterfield - 1910 - 424 pages
...information, appears somewhat more probable as an approximation / 0 \ to the truth. The ratio I k or -p- I of the specific heat at constant pressure to that at constant volume has been found by Maneuvrier and Fournier to be 1-26 ; but they did not measure the specific heat itself.*... | |
 | George William Clarkson Kaye, Thomas Howell Laby - 1911 - 172 pages
...| N, c» = 475 + •00016/, / being the temp. RATIO OF THE SPECIFIC HEATS FOR GASES AND VAPOURS 7 = the ratio of the specific heat at constant pressure to that at constant volume, у is usually determined directly by some method involving an adiabatic expansion, such as the determ1nat2on... | |
 | George William Clarkson Kaye, Thomas Howell Laby - 1911 - 172 pages
...|| N, ev = '175 + '00016/, / being the temp. RATIO OF THE SPECIFIC HEATS FOR GASES AND VAPOURS 1 = the ratio of the specific heat at constant pressure to that at constant volume, y is usually determined directly by some method involving an adiabatic expansion, such as the determination... | |
 | Alexander Graham Clark - 1911 - 472 pages
...heat of a gas ? Show that the value of n in an equation of the curve for an adiabatic expansion is the ratio of the specific heat at constant pressure to that at constant volume. (7) Combine Boyle's and Charles's laws. CHAPTER VIII HORSE-POWEK 82. What is Horse-power ? — -Considerable... | |
| |